beetrootMH Value Proposition
What’s the problem?
Lithium and valproate are two carefully controlled drugs which can have potentially harmful effects on patients who are prescribed them, both when they take the drugs and when they’re prescribed them but don’t take them.
How do we alleviate this problem?
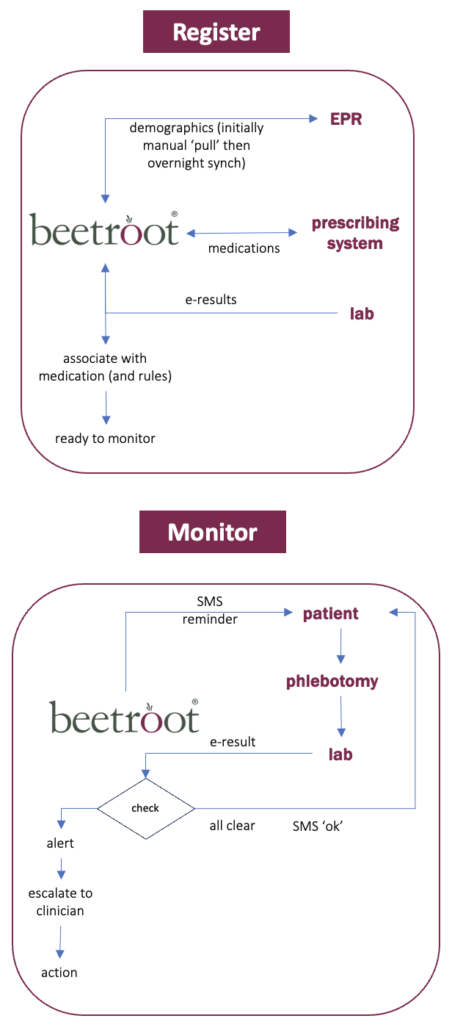
By deploying beetrootMH, a digital health monitoring service that enables the automation and digitisation of monitoring and audit of follow-up patients. An administrator is used to run the system rather than more expensive clinical staff.
beetrootMH both helps to ensure patient safety and enables compliance with NICE guidelines which are clear on advising monitoring frequencies:
How should I monitor someone taking lithium?
- Lithium levels are normally measured one week after starting treatment, one week after every dose change, and weekly until the levels are stable. Once levels are stable, levels are usually measured every 3 months.
- Lithium levels should be measured 12 hours post-dose.
- Measure weight or Body Mass Index (BMI), urea and electrolytes estimated glomerular filtration rate (eGFR), calcium and thyroid function tests every 6 months (more often if there is evidence of impaired renal function).
- If the person’s urea, or creatinine levels become elevated or if the eGFR declines over two or more tests, consider measuring lithium levels more frequently than 3 monthly. For more information, see the CKS topic on Chronic kidney disease.
- If there is a risk factor for, or existing, cardiovascular disease, an electrocardiogram (ECG) is normally performed before treatment begins.
[Taylor et al, 2012; NICE, 2018a]
How should I monitor someone taking valproate?
- Before starting treatment, a full blood count, baseline liver function tests (LFTs), and body weight or body mass index (BMI) are usually measured.
- Ensure that LFTs, BMI, and a full blood count are measured 6 months after treatment has been initiated, and every 12 months thereafter.
- Valproate levels are not routinely measured unless there is evidence of ineffectiveness, poor compliance, or toxicity is suspected.
[NICE, 2018a; Joint Formulary Committee, 2019]
How do we do this?
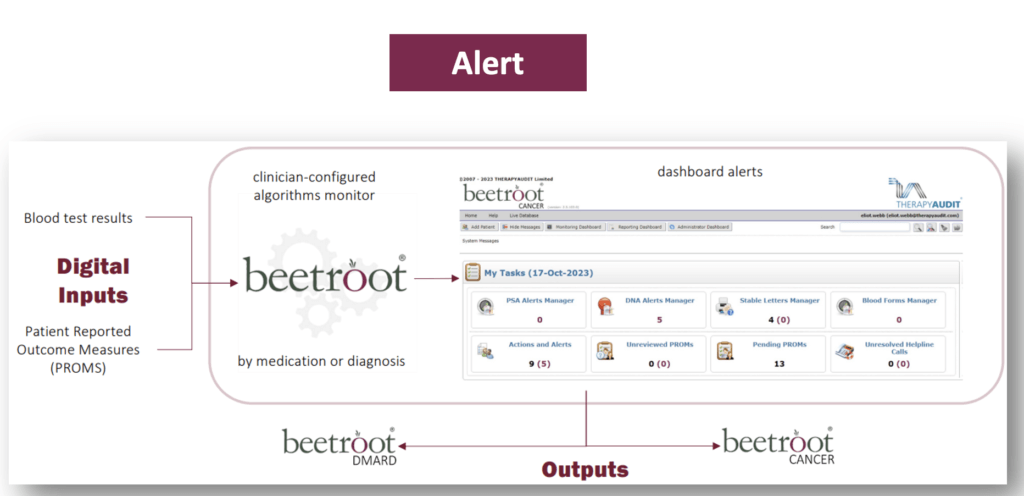
By enabling administrators on behalf of clinicians to set up digital monitoring pathways in beetroot and automatically collect and check relevant test results’ values from pathology.
beetrootMH provides dashboard-accessible alerts under the following circumstances:
- If any result is outside ‘normal’ rages.
- If any result indicates a deteriorating trend when compared to historic results.
- If a result hasn’t been received for a patient when expected (DNA).
Monitoring pathways can generate for patients SMS reminders to go for testing and SMS messages if results are normal. Additionally questionnaires (PROMS) can be automatically scheduled to be sent to patients for a further level of monitoring.
beetroot can also generate and track Shared Care Agreements used between secondary and primary care.
See the following slide for a simple explanation and sample screens from beetroot.